Monster Gym | bodybuilding gym UK, flexible gym membership UK, MMA & Boxing UK, Cardio Gym buy clenbuterol gel australia bodybuilding and it is benefits – biceps muscle
GMP API MANUFACTURING



Previous
Next
cGMP API MANUFACTURING
cGMP API MANUFACTURING

Stegram Pharmaceuticals is a leader in the development and manufacture of complex cGMP Active Pharmaceutical Ingredients (APIs). Our skilled scientists and recently updated and renovated cGMP development and manufacturing facility support the Drug Discovery process through API Synthesis for all stages of pre-clinical and clinical trials as well as small scale commercial manufacturing. We conduct GMP manufacturing of API’s from grams to multi-kilos. Our R&D and GMP manufacturing areas can support reactor sizes up to 300L.
Custom Synthesis of Small Molecules and biopolymers is carried out in our modern cGMP API manufacturing facility. Our two clean API suites are rated at Class 10,000 (Class C). We are currently involved in the small scale cGMP contract manufacturing of active pharmaceutical ingredients in many drug classes. Many of the APIs we currently manufacture started with us as a Contract Research Project or as Custom Synthesis Projects. Our integrated services allow our customers to move their API products from inception all the way through to cGMP manufacturing all in one company. Stegram Pharmaceuticals brings more than 30 years of chemistry experience to all API synthesis projects and we ensure cGMP compliance with our rigorous quality systems.
API Synthesis and Process Validation

Stegram Pharmaceuticals is a leader in the development and manufacture of complex cGMP Active Pharmaceutical Ingredients (APIs). Our skilled scientists and recently updated and renovated cGMP development and manufacturing facility support the Drug Discovery process through API Synthesis for all stages of pre-clinical and clinical trials as well as small scale commercial manufacturing. We conduct GMP manufacturing of API’s from grams to multi-kilos. Our R&D and GMP manufacturing areas can support reactor sizes up to 300L.
Custom Synthesis of Small Molecules and biopolymers is carried out in our modern cGMP API manufacturing facility. Our two clean API suites are rated at Class 10,000 (Class C). We are currently involved in the small scale cGMP contract manufacturing of active pharmaceutical ingredients in many drug classes. Many of the APIs we currently manufacture started with us as a Contract Research Project or as Custom Synthesis Projects. Our integrated services allow our customers to move their API products from inception all the way through to cGMP manufacturing all in one company. Stegram Pharmaceuticals brings more than 30 years of chemistry experience to all API synthesis projects and we ensure cGMP compliance with our rigorous quality systems.
API Synthesis and Process Validation
At Stegram Pharmaceuticals Quality and Compliance are very important to us. We pay special attention to equipment qualification and process validation and provide full regulatory support for our cGMP API manufacturing clients. We follow the strictest guidelines, perform the most rigorous testing and execute studies in a timely fashion to ensure that your product is of the highest quality when it reaches its destination.
Stegram Pharmaceuticals can develop, create and execute validation protocols and studies required for your products and equipment in accordance with current Guidelines (U.S. FDA, Health Canada (HPFBI), EMA, ICH, WHO) and acceptable formats (prospective, retrospective and concurrent). These include process validation, cleaning validation, aseptic processing, terminal sterilization, environmental control, IQ/OQ/PQ/PV and utility qualifications. Statistical analysis and decision making analysis are also part of our service. We can accelerate your Drug Development Program by integrating process development, cGMP API manufacturing and finish dose manufacturing all at a single location.
cGMP Sterile API Manufacturing
cGMP Sterile API Manufacturing
Stegram Pharmaceuticals offers the manufacturing of sterile cGMP APIs by a variety of methods including sterile filtration and dry heat sterilization. Our sterile product manufacturing capabilities include sterile filling of powders, liquids and lyophilization. Production of sterile APIs is a highly specialized capability not widely available in the industry. The manufacture of sterile API’s must be strictly controlled in order to minimize the risk of contamination with micro-organisms, endotoxins and particles. At Stegram Pharmaceuticals, we have successfully managed to develop compliant sterile APIs for a wide range of molecule types and applications.
Our manufacturing, packaging and testing for sterile APIs follows GUI-0104 “Good Manufacturing Practices (GMP) Guidelines for Active Pharmaceutical Ingredients (API) and ICH Q7 GMP’s for APIs. Our sterile API teams have more than a decade of experience with creating the appropriate conditions for the media fill and process validation. They are highly flexible and adaptable and will work with you throughout development and scale-up to manufacturing of your sterile API. Our manufacturing, designed and validated for sterile operations, uses a system of airlocks and cleanroom zones from class D to class A.


Stegram Pharmaceuticals offers the manufacturing of sterile cGMP APIs by a variety of methods including sterile filtration and dry heat sterilization. Our sterile product manufacturing capabilities include sterile filling of powders, liquids and lyophilization. Production of sterile APIs is a highly specialized capability not widely available in the industry. The manufacture of sterile API’s must be strictly controlled in order to minimize the risk of contamination with micro-organisms, endotoxins and particles. At Stegram Pharmaceuticals, we have successfully managed to develop compliant sterile APIs for a wide range of molecule types and applications.
Our manufacturing, packaging and testing for sterile APIs follows GUI-0104 “Good Manufacturing Practices (GMP) Guidelines for Active Pharmaceutical Ingredients (API) and ICH Q7 GMP’s for APIs. Our sterile API teams have more than a decade of experience with creating the appropriate conditions for the media fill and process validation. They are highly flexible and adaptable and will work with you throughout development and scale-up to manufacturing of your sterile API. Our manufacturing, designed and validated for sterile operations, uses a system of airlocks and cleanroom zones from class D to class A.
cGMP API Customers
cGMP API Customers

Bio provides GMP API manufacturing to meet regulatory requirements including Various UK Health Organizations and Health Care Centers, U.S. FDA, Health Canada and EMA standards.
The following quotes are examples of the feedback we have received from our API customers.
“I was most impressed by your operation from both a GMP and regulatory perspective.”
– GMP Compliance Manager, Corporate Quality
“I was particularly impressed with the knowledge of the staff that were present during the visit and the continuous improvement approach….”
– Director of Quality and QP
“There is a very knowledgeable team at Stegram Pharmaceuticals. The level of GMP compliance of the facility was considered satisfactory for the continued manufacture of API and for Drug Products to be imported to EU.”
– CEO, QP
“….Stegram Pharmaceuticals had a well-functioning and effective quality system
….All involved personnel gave a very competent impression.”
– ISO/GMP Lead Auditor

Bio provides GMP API manufacturing to meet regulatory requirements including Various UK Health Organizations and Health Care Centers, U.S. FDA, Health Canada and EMA standards.
The following quotes are examples of the feedback we have received from our API customers.
“I was most impressed by your operation from both a GMP and regulatory perspective.”
– GMP Compliance Manager, Corporate Quality
“I was particularly impressed with the knowledge of the staff that were present during the visit and the continuous improvement approach….”
– Director of Quality and QP
“There is a very knowledgeable team at Stegram Pharmaceuticals. The level of GMP compliance of the facility was considered satisfactory for the continued manufacture of API and for Drug Products to be imported to EU.”
– CEO, QP
“….Stegram Pharmaceuticals had a well-functioning and effective quality system
….All involved personnel gave a very competent impression.”
– ISO/GMP Lead Auditor
API Process Development Services
API Process Development Services
Stegram Pharmaceuticals is an ideal contract partner for your API Process Development. We are FDA registered and Health Canada approved, and provide integrated process development, API manufacturing and finished dose manufacturing at a single location. We have all the expertise required for developing a process that is robust, transferable, and scalable to meet your requirements. We bring 30 years of experience to every project and deliver fully integrated solutions with an emphasis on quality, speed, and flexibility.
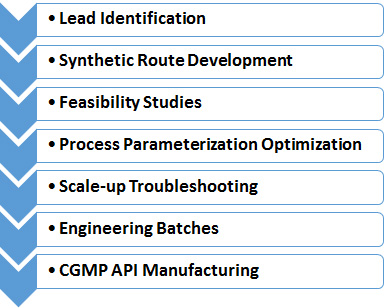
Our Process Development and Manufacturing Services include:
- Custom Synthesis from mg to multi-kilogram scale in batch (up to 300L reactors) or continuous process
- Optimization of existing synthetic routes
- Development of GMP friendly alternative synthetic routes
- Development of IP protected synthetic routes
- Statistical Design of Experiments (DoE) for process parameterization and optimization
- Development and optimization of purification and isolation methods
- Isolation of active ingredients from natural sources
- Transfer of process into GMP
- Troubleshooting
- Polymorphism study
- Documentation of CMC development projects

Our Process Development and Manufacturing Services include:
- Custom Synthesis from mg to multi-kilogram scale in batch (up to 300L reactors) or continuous process
- Optimization of existing synthetic routes
- Development of GMP friendly alternative synthetic routes
- Development of IP protected synthetic routes
- Statistical Design of Experiments (DoE) for process parameterization and optimization
- Development and optimization of purification and isolation methods
- Isolation of active ingredients from natural sources
- Transfer of process into GMP
- Troubleshooting
- Polymorphism study
- Documentation of CMC development projects
We support clients in all aspects of API Process Development, including process and analytical R&D, stability studies, impurity/degradant profiles, and raw material sourcing. Dalton understands your need for efficient outsourcing solutions in process development and is focussed on reducing timelines and costs throughout all phases of development and manufacturing.

Stegram pharmaceutical
72 high Beeches, Banstead, surrey,Sm7 1NW United Kingdom.
reach us at : [email protected]
Phone: +44(0)8712455161
WHO WE ARE
Our Profile
Mission Statement
Company Facilities
WHAT WE DO
cGMP API Manufacturing
Formulation develoopment ……
Custom synthesis and ……
Contract analytical testing ……
Contract Research
Products
Prescription medicines
Consumer Health products
Safety of medicines
Quality control
Our vetirinary products
Counterfiet medicines
Reporting side effects

72 high Beeches, Banstead, surrey,Sm7 1NW United Kingdom.
reach us at : [email protected]
Phone: +44(0)8712455161
WHO WE ARE
Our Profile
Mission Statement
Company Facilities
WHAT WE DO
cGMP API Manufacturing
Formulation develoopment ……
Custom synthesis and ……
Contract analytical testing ……
Contract Research
PRODUCTS
Prescription medicines
Consumer Health products
Safety of medicines
Quality control
Our vetirinary products
Counterfiet medicines
Reporting side effects
copyright© 2022 stegrampharma.co.uk
