CUSTOM SYNTHESIS AND FINE CHEMICAL
CUSTOM SYNTHESIS AND FINE CHEMICAL
CUSTOM SYNTHESIS AND FINE CHEMICAL
Custom Synthesis And Fine Chemical
Custom Synthesis And Fine Chemical
Custom Synthesis And Fine Chemical

Custom synthesis is the process where a molecule is made exclusively for a particular client according to specifications at their scale. It is one of the options available for pharmaceutical companies requiring active pharmaceutical ingredients (APIs), intermediates, fine chemicals, reference standards, impurities or metabolites needed to launch their research and development projects. Custom synthesis is essential to the pharmaceutical companies since many of these compounds can sometimes be very difficult to find or hard to synthesize. Custom synthesis provides the platform necessary for clients’ to accelerate their projects to market.
Stegram Pharmaceuticals Custom Synthesis and Fine Chemical services offers a wide range of experience in the synthesis of low volume, high value small molecules for biotechnology and pharmaceutical applications. With services available in vessels up to 300L in size and R&D and cGMP environments, Stegram Pharmaceuticals can meet your custom synthesis and fine chemicals requirements.

Custom synthesis is the process where a molecule is made exclusively for a particular client according to specifications at their scale. It is one of the options available for pharmaceutical companies requiring active pharmaceutical ingredients (APIs), intermediates, fine chemicals, reference standards, impurities or metabolites needed to launch their research and development projects. Custom synthesis is essential to the pharmaceutical companies since many of these compounds can sometimes be very difficult to find or hard to synthesize. Custom synthesis provides the platform necessary for clients’ to accelerate their projects to market.
Stegram Pharmaceuticals Custom Synthesis and Fine Chemical services offers a wide range of experience in the synthesis of low volume, high value small molecules for biotechnology and pharmaceutical applications. With services available in vessels up to 300L in size and R&D and cGMP environments, Stegram Pharmaceuticals can meet your custom synthesis and fine chemicals requirements.
Custom Synthesis has been a specialty since the inception of Stegram Pharmaceuticals , and we are constantly advancing our capabilities and chemistry support services. Where appropriate, we use Flow Chemistry Reactor Technology for custom synthesis projects. Potential benefits for our clients include increased scalability, flexibility, reaction speed, process control, and cost savings.
Stegram Pharmaceuticals specializes in the custom synthesis of a wide range of small molecules including active pharmaceutical ingredients, pharmaceutical intermediates, drug intermediates, drug metabolites, drug analogs, analytical standards, reference standards and certified reference materials, chemical intermediates, chemical standards, and pharmaceutical impurities.
Our extensive experience in synthetic organic chemistry, including complex chemistry, chiral compounds, hydrogenation, natural products chemistry, organometallic chemistry, nucleotide and amino acid production, stable isotope synthesis, fluorescent probes, and inert atmosphere chemistry sets us apart from the competition. We also offer specialized synthesis of controlled drug classes including Steroids, Opiods and Cannabinoids.
We excel at process development, process improvement, and isolation of biologically active molecules from natural sources. In addition, Stegram Pharmaceuticals custom synthesis department is supported by our in-house analytical chemistry laboratory offering a full service GMP testing environment. For clients requiring peptides, we have a Custom Peptide Synthesis laboratory.
For product details on our custom synthesis and fine chemicals, please check our Stegram Pharmaceuticals Research Molecules Catalog.
Flow Chemistry Services
Flow Chemistry Services
Flow Chemistry Services
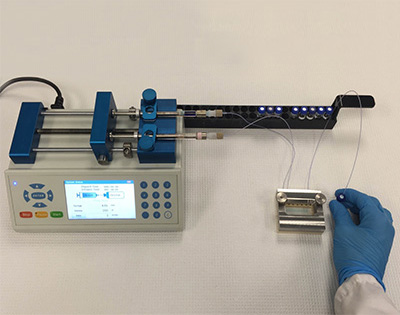
Flow Chemistry Reactor Technology is part of Stegarm Pharmaceuticals extensive arsenal of capabilities in the area of custom synthesis and process development. The many benefits of this exciting new technology include increased scalability, flexibility, and process control.
Flow Chemistry is the manufacture of compounds using a continuous process with automated pumps and specialized reactors. The reactants flow as solutions in these reactors, and mixing is completed in a fraction of a second rather than the much longer time frames associated with conventional batch processes. The reaction is finished when the solutions exit. Only a fraction of the total material used in API and custom synthesis reacts at any moment, and consequently reactor volume is minimized.

Flow Chemistry Reactor Technology is part of Stegarm Pharmaceuticals extensive arsenal of capabilities in the area of custom synthesis and process development. The many benefits of this exciting new technology include increased scalability, flexibility, and process control.
Flow Chemistry is the manufacture of compounds using a continuous process with automated pumps and specialized reactors. The reactants flow as solutions in these reactors, and mixing is completed in a fraction of a second rather than the much longer time frames associated with conventional batch processes. The reaction is finished when the solutions exit. Only a fraction of the total material used in API and custom synthesis reacts at any moment, and consequently reactor volume is minimized.
The high surface area to volume ratio in a small flow reactor allows for nearly instantaneous control over reaction temperatures, giving potentially cleaner products and increased reaction safety. Scale-up issues are minimized through the more consistent control of mixing and heat transfer throughout the reaction at any scale, even up to a tonne. Flow Chemistry also facilitates a faster change-over between the different custom synthesis projects.
Flow chemistry is chosen as an alternative approach to batch synthesis when it is likely to deliver better yields, faster reactions, or safer operations. It is also selected to access novel or exotic synthesis routes which would be impossible or impractical using batch technology
Examples of Custom Synthesis in which Flow Chemistry might be the best option are:
Reactions needing fast mixing for good selectivity
Very exothermic or endothermic reactions
Reactions requiring high pressures or temperatures
Reactions with gas release
Scale-up of micro-wave chemistry
Reactions with reactants/catalysts in an immiscible phase (liquid-liquid or gas-liquid)
Initial investigation and proof of concept of Custom Synthesis at Stegram Pharmaceuticals is usually done on the milligram scale using a Chemtrix micro-reactor. A full experimental program, using very little material, to determine kinetics of the reaction and the effects of various operating conditions can usually be done in 1-3 days. When output of more than just a few milligrams of product is desired, the process is scaled-up using in-house flow reactors. Because of the nature of Flow Chemistry, there is minimal troubleshooting. Overall, Flow Chemistry allows Stegram Pharmaceuticals to provide all of its clients with a more cost-effective, efficient and safer approach for API process development and custom synthesis.
Conjugation Services
Conjugation Services
Conjugation Services

Stegram Pharmaceuticals is a recognized leader in offering custom Conjugation Services as a tool for Drug Delivery, Targeted Drug Delivery, Synergistic Drug and Gene Delivery, or Diagnostic applications.
Stegram Pharmaceuticals technical experts function as an effective extension of your drug development team. We offer customized design & synthesis solutions for the conjugation of small molecules such as drugs, metabolites, and labelled compounds with synthetic or natural polymers for specific applications. Applying specialized analytical tools, our scientists can develop analytical methods to characterize your drug conjugates.
Our conjugation services include:
- Conjugated antibodies
- Conjugated polymers
- Conjugated proteins
- Conjugation of fluorophores
- Biotin conjugation
- Antibody-drug conjugate (ADC)
- Polymer–drug conjugation
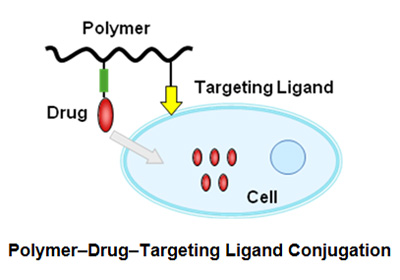
- Polymer-drug-target ligand conjugation
- Polymer–radioligand–target ligand conjugation

Stegram Pharmaceuticals is a recognized leader in offering custom Conjugation Services as a tool for Drug Delivery, Targeted Drug Delivery, Synergistic Drug and Gene Delivery, or Diagnostic applications.
Stegram Pharmaceuticals technical experts function as an effective extension of your drug development team. We offer customized design & synthesis solutions for the conjugation of small molecules such as drugs, metabolites, and labelled compounds with synthetic or natural polymers for specific applications. Applying specialized analytical tools, our scientists can develop analytical methods to characterize your drug conjugates.
CONJUGATION
Conjugation is the process of covalently linking two molecules/polymers or biomolecules/biopolymers together. Conjugation of small molecule therapeutics to polymers can enhance efficacy by improving pharmacokinetic and pharmacodynamic properties by reducing plasma protein binding, reducing toxicity, or by improving stability.
Drug conjugates are being developed for a range of therapeutic indications including cardiovascular disease, acute invasive fungal infections, immune modulation, and more. Conjugation is also being explored as a means of synergistic drug and gene delivery by conjugating a drug with a cationic polymer such as PEI, which is capable of complexing with negatively charged DNA. The polyplexes formed, then release the DNA and drug molecule upon internalization into the cell.
TARGETED DRUG DELIVERY
Research is ongoing for the development of new drug delivery systems for targeted drug delivery. Equipping polymer-drug conjugates with target cell specific ligands like EGF and RGD peptides can provide a solution for selective and targeted delivery.
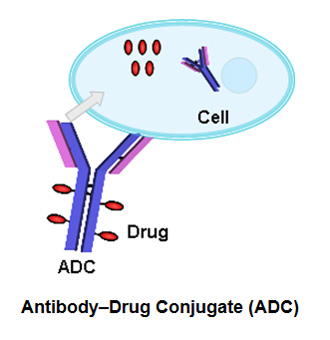
The largest class of conjugated drug products under development is the antibody-drug conjugate (ADC). Antibody-drug conjugates combine a drug with a monoclonal antibody which provides selective targeting. The drug may be released by enzymatic cleavage of the linker.
TARGETED IMAGING
The conjugation approach is being strategically investigated for targeted imaging to achieve a large contrast enhancement at the diseased site. Conjugation of a targeting ligand such as RGD or EPR and a radio-ligand chelator such as DOTA with a polymer has added a new dimension to diagnostic imaging (PET, MRI etc.) for targeted tumor mapping.
FLUOROPHORES
Stegram Pharmaceuticals Limited offers the conjugation of a fluorophore (e.g. labeled with fluorescein, rhodamine, biotin or avidin) with synthetic polymers or biopolymers for use as a tool in molecular biology or for the study of drug modes of action.
The Dalton Edge
ANALYTICAL
Stegram Pharmaceuticals has the analytical tools and experience that are critical for this field. The analytical requirements for supporting the manufacture, testing as well as regulatory safety and efficacy of drug conjugates are technically challenging. The biological or polymeric carrier can be coupled to the drug through site-specific linking or through random linking (involving multiple sites of similar reactivity). The stoichiometry (i.e. the mean ratio of the carrier to the drug) may reasonably characterize the conjugate in the case of site-specific linking. When the linking is random, the product is not homogeneous, and the mean ratio is a reflection of the distribution of conjugate subpopulations.
The conjugation reaction may produce by-products, or may be incomplete, leaving residues of carrier, drug, and linker. Sophisticated analytical methods must be used to follow the often difficult purification steps. Sensitive analytical tools are needed to investigate stability, to characterize the end product, and to ensure batch to batch consistency.
SMALL MOLECULE AND BIOLOGICAL EXPERTISE
Conjugation leverages Stegram Pharmaceuticals extensive experience in both small molecules and large biopolymers. This includes synthesis, analysis, purification, cGMP manufacturing, and aseptic filling (see next section).
GMP ASEPTIC FILLING
Conjugation products developed at Stegram Pharmaceuticals can be seamlessly prepared for preclinical and early stage clinical trials in our fully integrated GMP Class 100 Aseptic Filling Manufacturing Facility. Our capability includes sterile filling of liquid-in-vial injectable drugs, aseptic filling and stoppering of liquid prefilled syringes, and aseptic filling and capping of powders. We provide our clients with full regulatory compliance support. Stegram Pharmaceuticals has the capabilities to develop, create, and execute validation protocols in accordance with current Guidelines (HPFBI, U.S. FDA, EMA, ICH, WHO) and acceptable formats (prospective, retrospective, and concurrent).
- Sterile Liquid Injectables: validated for 3, 5, and 20 ml vials up to 15,000 units per run.
- Aseptic Powder Fill: validated for 20 ml vials up to 10,000 units per run.
How Dalton Works with Clients
In the typical arrangement, the client identifies the drug and the linkage requirement, and provides the carrier molecule. In some cases the client will provide a proprietary drug. Pharmaceuticals obtains or synthesizes the required link, develops the linkage method, uses appropriate analytical tools to characterize the product, and works with the client to optimize the drug conjugate for the intended clinical use.
Custom Peptide Synthesis
Custom Peptide Synthesis
Custom Peptide Synthesis

Our Custom Peptide Synthesis laboratory can produce and purify synthetic peptides in either R&D or GMP environments, and aseptically fill the peptides in our cGMP facility*. Our peptide synthesis service includes the manufacture of normal peptides and custom modified peptides up to 12-15 amino acids in length to suit your requirements.
Solid phase peptide synthesis or solution phase chemistry can be used to manufacture your custom peptides. Pre- or post-synthesis operations can incorporate natural amino acids, modified amino acids, and unnatural amino acids. Post synthesis modifications include biotinylation, peptide conjugation, KLH conjugation, linker attachment, and more.
We use preparative HPLC to isolate the desired peptide, and the final compound is analyzed again by reverse phase HPLC to verify purity. Depending on your research needs, Stegram Pharmaceuticals can provide material in a range of peptide purity from 70-98%. Mass spectrometry is used to verify the mass of the peptide.
Purification can be rapidly completed with our range of HPLC systems with a 200ml/min flow rate. We have HPLC columns with capacities up to 2.2 liters.

Our Custom Peptide Synthesis laboratory can produce and purify synthetic peptides in either R&D or GMP environments, and aseptically fill the peptides in our cGMP facility*. Our peptide synthesis service includes the manufacture of normal peptides and custom modified peptides up to 12-15 amino acids in length to suit your requirements.
Solid phase peptide synthesis or solution phase chemistry can be used to manufacture your custom peptides. Pre- or post-synthesis operations can incorporate natural amino acids, modified amino acids, and unnatural amino acids. Post synthesis modifications include biotinylation, peptide conjugation, KLH conjugation, linker attachment, and more.
We use preparative HPLC to isolate the desired peptide, and the final compound is analyzed again by reverse phase HPLC to verify purity. Depending on your research needs, Centric Pharmaceuticals Limited can provide material in a range of peptide purity from 70-98%. Mass spectrometry is used to verify the mass of the peptide.
Purification can be rapidly completed with our range of HPLC systems with a 200ml/min flow rate. We have HPLC columns with capacities up to 2.2 liters.
We are proud of the knowledge and expertise that enables us to offer custom peptide synthesis services to our clients. Stegram Pharmaceuticals is recognized for high quality and technical service, and we stand by what we produce. Our peptides are manufactured to the highest quality standards here in our North American facilities.
* With our new 18 sq ft lyophilizer, on stream in 2020, we have the capacity for aseptic filling up to 6,750 vials of lyophilized peptide (in 3 ml vials; other vial sizes are available).
GMP Aseptic Filling of Oligonucleotides
GMP Aseptic Filling of Oligonucleotides
GMP Aseptic Filling of Oligonucleotides

Our capabilities for GMP Aseptic Filling of Oligonucleotides will support your pharmaceutical drug development process as it advances through clinical trials and on to commercial production. Our pharmaceutical cGMP Manufacturing Facilityhandles both aseptic liquid filling and aseptic powder filling into vials. Stegram Pharmaceuticals uses peristaltic filling equipment with low hold up volumes to ensure that losses on filling your valuable drug product are reduced.
The aseptic fill suite maintains a local Grade A (ISO Class 5) environment for the filling operations. Our Microbiology Laboratory routinely monitors all areas of our cGMP aseptic processing facility for viable airborne contamination, viable contamination on surfaces and non-viable airborne contamination.
We are flexible and committed to the success of your oligonucleotide program and pharmaceutical drug development program. While other sterile fill companies may impose a requirement for a minimum number of vials, Dalton welcomes small scale aseptic fills to move you quickly into the clinic and help you achieve key milestones.
Stegram Pharmaceuticals offers fully integrated GMP Aseptic Filling and capping of liquid in-vial injectable drugs, and aseptic powder filling and capping for preclinical and early stage clinical trials.

Our capabilities for GMP Aseptic Filling of Oligonucleotides will support your pharmaceutical drug development process as it advances through clinical trials and on to commercial production. Our pharmaceutical cGMP Manufacturing Facilityhandles both aseptic liquid filling and aseptic powder filling into vials. Centric Pharmaceuticals Limited uses peristaltic filling equipment with low hold up volumes to ensure that losses on filling your valuable drug product are reduced.
The aseptic fill suite maintains a local Grade A (ISO Class 5) environment for the filling operations. Our Microbiology Laboratory routinely monitors all areas of our cGMP aseptic processing facility for viable airborne contamination, viable contamination on surfaces and non-viable airborne contamination.
We are flexible and committed to the success of your oligonucleotide program and pharmaceutical drug development program. While other sterile fill companies may impose a requirement for a minimum number of vials, Dalton welcomes small scale aseptic fills to move you quickly into the clinic and help you achieve key milestones.
Stegram Pharmaceuticals offers fully integrated GMP Aseptic Filling and capping of liquid in-vial injectable drugs, and aseptic powder filling and capping for preclinical and early stage clinical trials.
Sterile Liquid Injectables: Aseptic fill on a state-of-the-art fill / finish crimp-cap line, capable of aseptic filling vials from 1 to 125 ml. We have validated processes in place for sterile filling 2, 3, 5, and 20 ml vials up to 15,000 units per run.
Supporting our process equipment are validated services including a WFI system, pure steam generator, autoclave and depyrogenation oven. Validation capabilities include, but are not limited to, aseptic fill simulation studies, terminal sterilization loads, autoclave loads, depyrogenation oven loads, container closure integrity and process validation. Stegram Pharmaceuticals also has the ability to develop or transfer technology related to aseptic processing. In addition, Stegram Pharmaceuticals analytical service laboratory offers method development, validation and ICH stability programs to its clients. QC/QA and Microbiology functions support our sterile manufacturing services.
Stegram Pharmaceuticals is currently manufacturing and filling sterile clinical trial products under cGMP. Our aseptic fill finish team will be an extension of your company and we will serve as a reliable and responsive partner to get you through the clinical trial process faster.
Integrated Drug Discovery, Development and Manufacturing

Stegram pharmaceutical
72 high Beeches, Banstead, surrey,Sm7 1NW United Kingdom.
reach us at : [email protected]
Phone: +44(0)8712455161

72 high Beeches, Banstead, surrey,Sm7 1NW United Kingdom.
reach us at : [email protected]
Phone: +44(0)8712455161
copyright© 2022 stegrampharma.co.uk
